What Does the Formula of an Ionic Compound Describe
ABa2N3 BBa2N2 CBa3N2 DBa3N4. The compound from the other ionic compounds containing the same elements.
Thus we must use the ratio of Na and Cl- ions the empirical formula to describe the compound.
. Ionic compounds like NaCl exist only as separate Na and Cl- ions in a crystalline arrangement. For example sodium chloride table salt Na Cl is an ionic compound. Here the sodium ion N a is a positive ion so it is attracted to the chlorine atom ion C l- which has a negative charge and the ionic bond is formed.
What does a compound formula tell us about the compound. Identify the formulas and charges of the cation and anion. Two-element ionic compounds include sodium chloride or table salt.
Two chloride ions were needed in the final compound because calcium had a 2 charge. SAP2 EU SAP2B LO SAP2B3 EK Transcript. If it is a molecular compound the formula indicates the numbers of atoms of.
One way of estimating the ionic character of a bondthat is the magnitude of the charge separation in a polar covalent bondis to calculate the difference in electronegativity between the two atoms. The formula of an ionic compound represents the lowest whole number ratio of cations to anions it is as simple as that. Its ionic formula is written as CaCl 2 the neutral combination of these ions.
The chemical formula that results upon the completion of this final step is a chemically-correct formula for an ionic compound. The empirical formula tells the lowest w. Therefore Be CN 2 is the chemical formula for the compound formed when the cyanide ion and beryllium bond with one another.
4 rows In the solid state ionic compounds are in crystal lattice containing many ions each of the. A compound made from only 2 elements. Bond polarity and ionic character increase with an increasing difference in electronegativity.
Tap again to see term. The formula does not indicate that a compound is composed of molecules. This is three positive charges and two negative charges.
These are metals and are placed on the left side of the periodic table. Ionic Compound Formula and the Principle of Charge Neutrality. What Does The Chemical Formula Of A Compound IndicateA chemical formula is an expression that shows the elements in a compound and the relative proportions of those elements.
What would be the chemical formula for the ionic compound barium nitride. Place the cation first in the formula followed by the anion. Choose the anion or the ion with the negative charge.
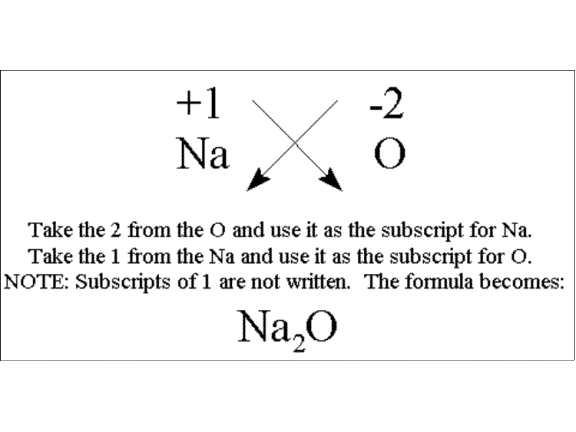
The formula of an ionic compound describes the ratio of the ions in the compound. Then identify the anion and write down its symbol and charge. Barium ions carry a 2 charge and nitrogen ions carry a 3- charge.
The symbols used to create a chemical formula are found on the periodic table. What must the name of an ionic compound distinguish. Beware that this must be the lowest whole ratio of cation to anion.
The opposite charges attract and the oppositely charged. This is so that the charges are balanced and the compound is neutral overall. Finally combine the two ions to.
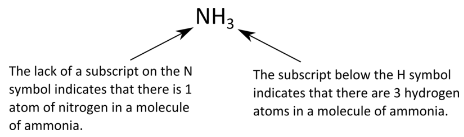
Determine how many of each ion type is needed to make a neutral compound. Formulas describe the ratio of atoms in a substance and the number of atoms in a single molecule of the substance thus the formula for ethene is C 2 H 4 rather than C H 2. Yes ionic compounds have only empirical formulas.
As for ionic compounds it tells us the number of atoms present in one formula unit of the compound. So the formula is. To create a neutral compound CaCl 2 two 1- chloride ions were needed to balance out the 2 charge from calcium.
Only separate sodium and chloride ions. For instanceH2O tells us that there are two hydrogen and one oxygen atom per molecule of water. Correctly order the steps followed in writing the formula of an ionic compound containing a polyatomic ion.
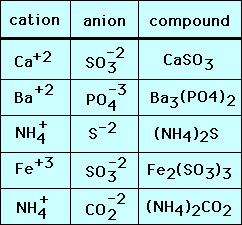
The chemical formula for the ionic compound K2S indicates that there isare ____ K ions for every ____ S2- ions. The ionic compound sodium chloride is made up of sodium cations Na and chloride anions Cl in a 11 ratio. Aluminium oxide contains Al3 and O2- ions.
The formula for an ionic compound must contain the same number of positive and negative charges. The ratio of the ions in the compound. Choose the cation or the ion with the positive charge.
Molecular formulas do not indicate how the atoms are arranged in the molecule. The formula of an ionic compound describes the ratio of the ions in the compound. What does the formula of an ionic compound describe.
What is a binary ionic compound. Click again to see term. To find the formula of an ionic compound first identify the cation and write down its symbol and charge.
The name of an ionic compound must distinguish the compound from other ionic compounds containing the same elements. The formula of an ionic compound is achieved by following the steps below. Δχ χB χA.
Since Na has an atomic mass of 23 u and Cl has an atomic mass of 355 u the formula mass for NaCl is 23355585 u. To balance we need two Al3 ions and three O2- ions. There are no molecules.

Writing Ionic Formulas Basic Introduction Youtube
No comments for "What Does the Formula of an Ionic Compound Describe"
Post a Comment